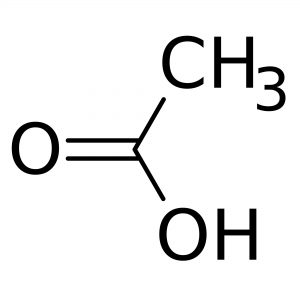
Methanol [67-56-1]
Methanol is produced industrially, starting with the production of syngas or syngas. The synthesis gas used in the production of methyl alcohol is a mixture of carbon monoxide and hydrogen formed when natural gas reacts with steam or oxygen. The methyl alcohol is then synthesized from carbon monoxide and hydrogen.
Boiling point: 64.5 ° C (1013 hPa)
Density: 0.792 g / cm3 (20 ° C)
Pairing number: 1.9
Explosive limits: 5.5 – 44% (V)
Flash point: 9.7 ° C
Auto-ignition temperature: 420 ° C DIN 51794
Melting point: -98 ° C
Vapor pressure: 128 hPa (20 ° C)
Hazard pictograms
Labeling of hazardous chemicals and mixtures that are part of the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). The pictograms recommended by GHS have the shape of a square set on the top. They should contain a black symbol on a white background with a red border.
Priority rules to be observed in connection with the labeling of a substance:
– the skull and crossbones, the exclamation mark pictogram should not be added.
– corrosive effect, the exclamation mark pictogram should not be added if it concerns eye or skin irritation.
– health hazard determining respiratory sensitization, the exclamation mark pictogram should not be added if it concerns skin sensitization or irritation to eyes or skin.
Source: GHS pictograms





Reviews
There are no reviews yet.